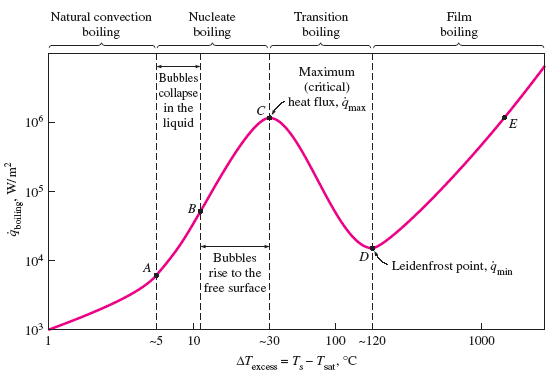
a) Martinelli parameter, (b) Boiling number, and (c) Weber number vs.... | Download Scientific Diagram
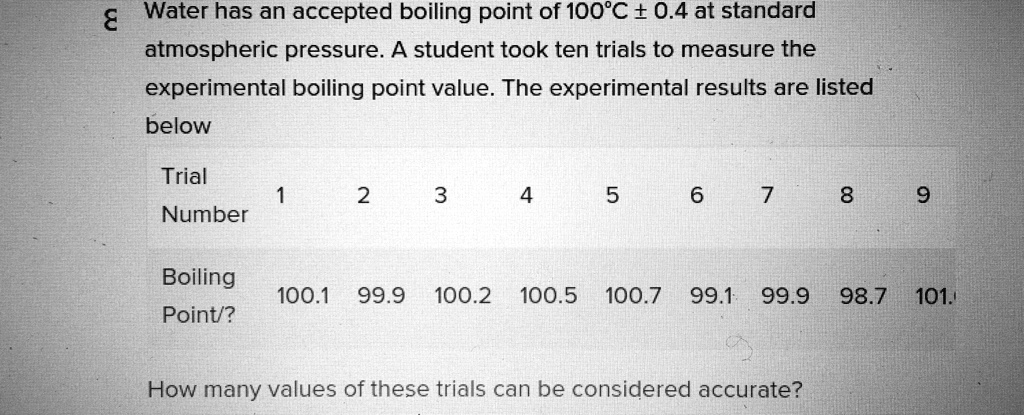
SOLVED: "How many values of these of these trials can be considered accurate? 8 Water has an accepted boiling point of 10O'C + 0.4 at standard atmospheric pressure. A student took ten
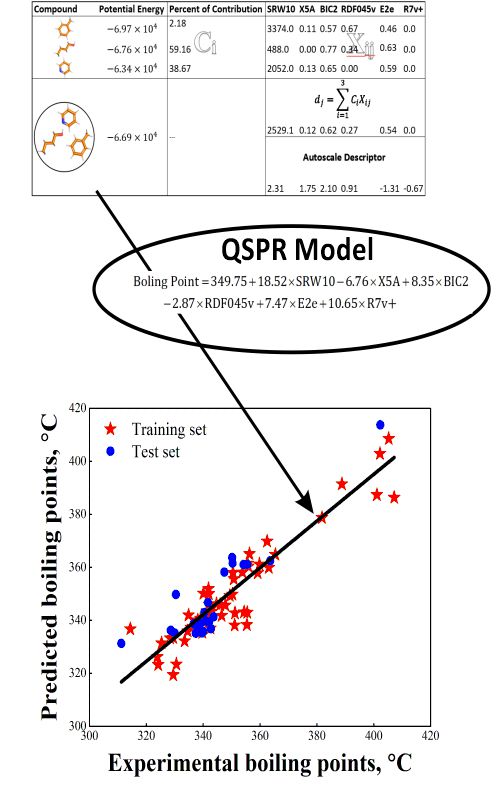
New structure-based models for the prediction of normal boiling point temperature of ternary azeotropes | Journal of the Serbian Chemical Society

Supercritical “boiling” number, a new parameter to distinguish two regimes of carbon dioxide heat transfer in tubes - ScienceDirect
![The boiling point of 0.1molal K4 [ Fe (CN) 6 ] solution will be : ( Given Kbfor water = 0.52K kg mol^-1 ) The boiling point of 0.1molal K4 [ Fe (CN) 6 ] solution will be : ( Given Kbfor water = 0.52K kg mol^-1 )](https://dwes9vv9u0550.cloudfront.net/images/10122715/8ce81e44-11b6-431d-9820-f76860bcff33.jpg)
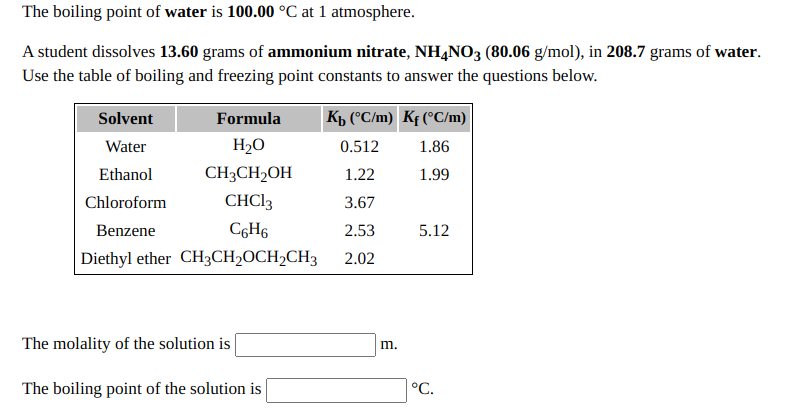
The boiling point of 0.1molal K4 [ Fe (CN) 6 ] solution will be : ( Given Kbfor water = 0.52K kg mol^-1 )

Supercritical “boiling” number, a new parameter to distinguish two regimes of carbon dioxide heat transfer in tubes - ScienceDirect