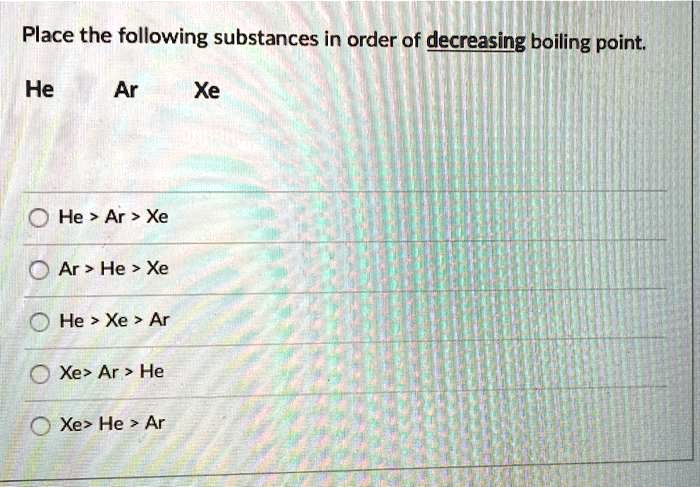
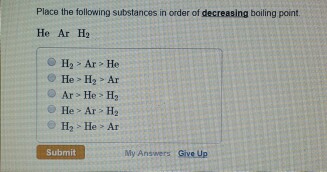
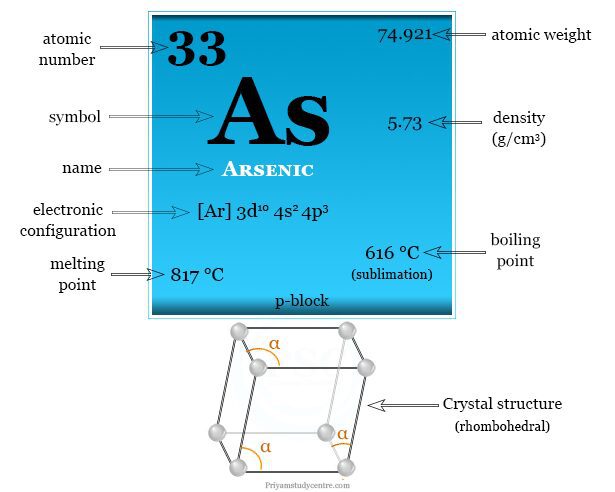
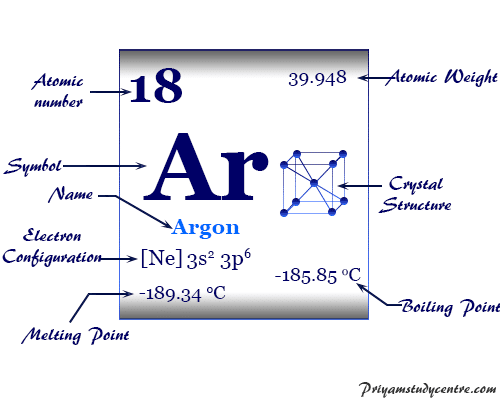
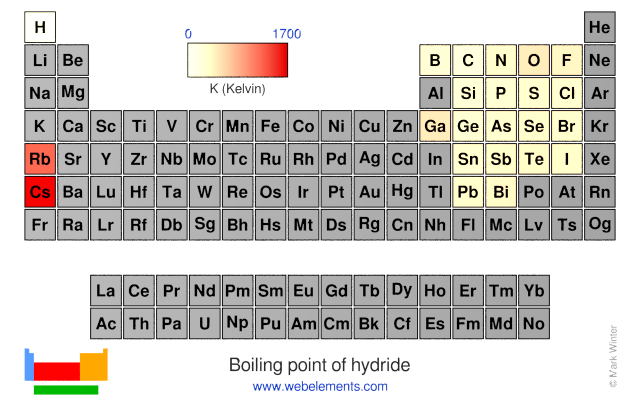
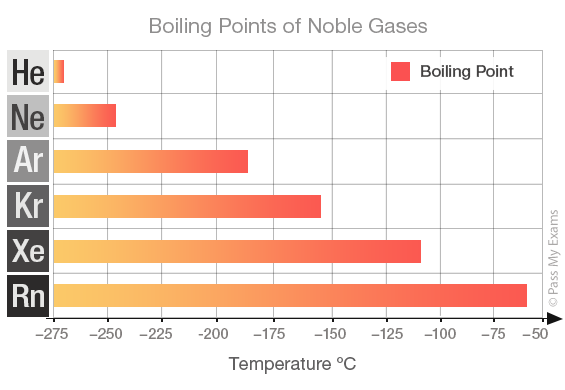
The correct order of boiling points of noble gases is | 12 | THE NOBLE GASES | CHEMISTRY | DINE... - YouTube

The Four Intermolecular Forces and How They Affect Boiling Points | Intermolecular force, Chemistry notes, Teaching tips

Elements General Physical Properties : Atomic Size | Melting point | Boiling point - The Chemistry Guru

inorganic chemistry - Why do the melting and boiling points of the noble gases increase when the atomic number increases? - Chemistry Stack Exchange
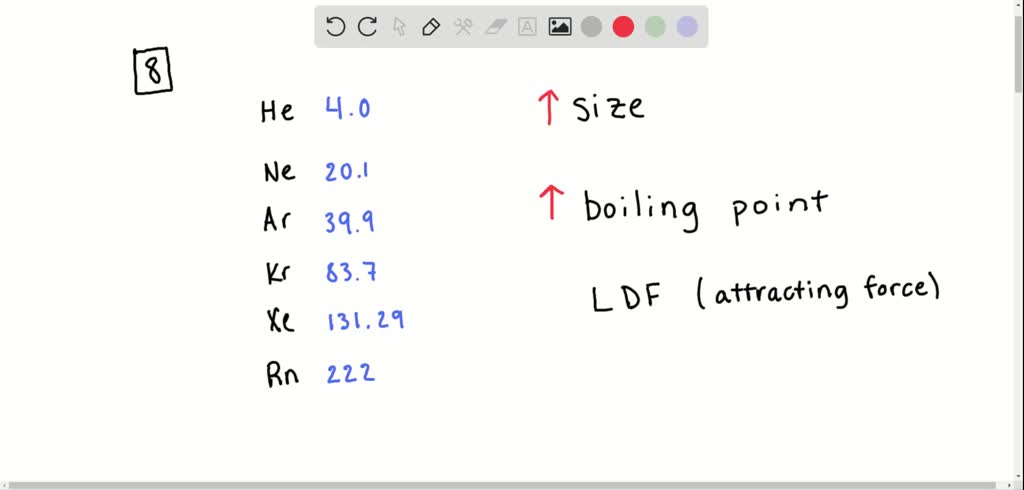
SOLVED:The boiling points of the noble gas elements are listed below. Comment on the trend in the boiling points. Why do the boiling points vary in this manner? He -272^∘C Kr -152.3^∘C
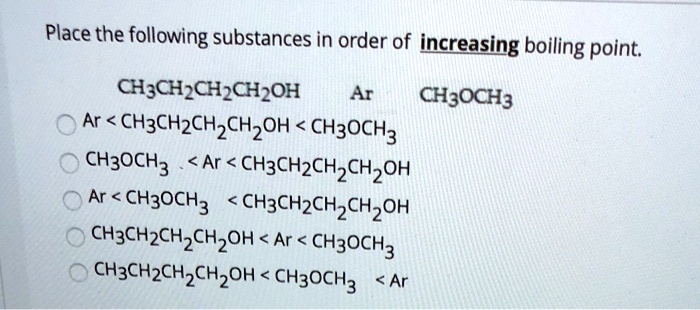
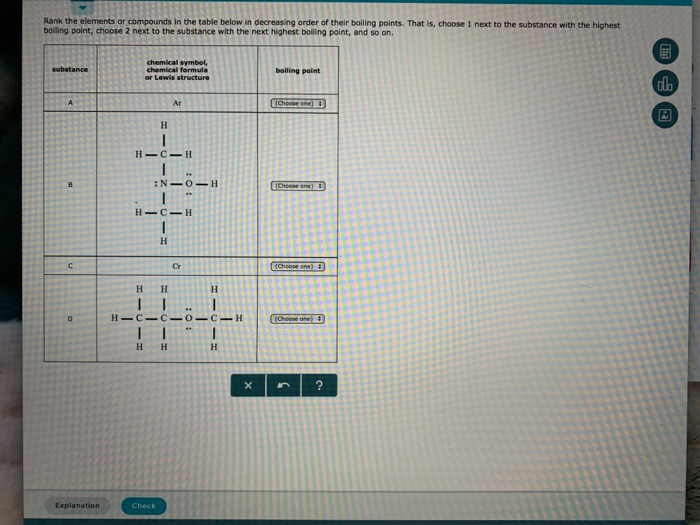
SOLVED: Place the following substances in order of increasing boiling point: CH;CHCHCHzOH Ar CH;OCH3 Ar < CH3CHzCHzCHzOH CH3OCHz CH3OCH3 Ar < CH3CHzCHzCHzOH Ar < CH3OCH3 CH3CHzCHzCHzOH CH3ChzCHzCHzoh <Ar < CH3OCH3 CHzCHzCHzCHzoh CH3OCH3 <Ar

Arrange the following in order of increasing boiling point MgCl2 He H2O CH3Cl Ar CO2 I know the cor - YouTube