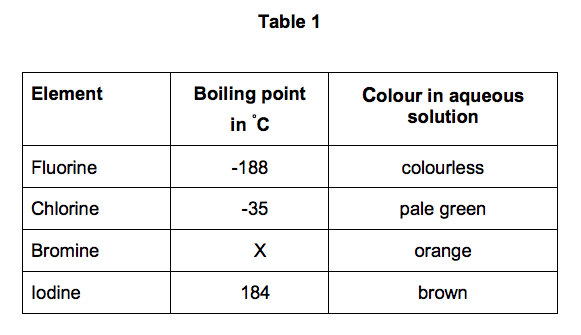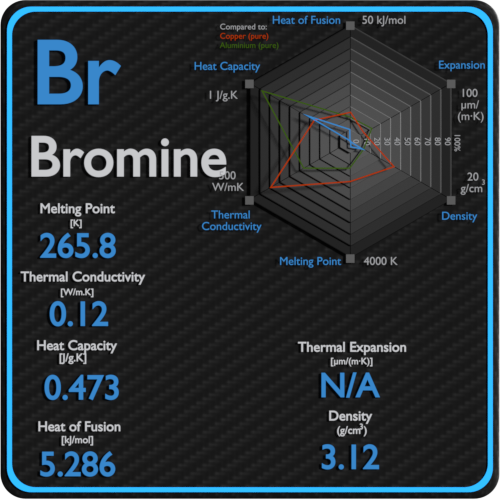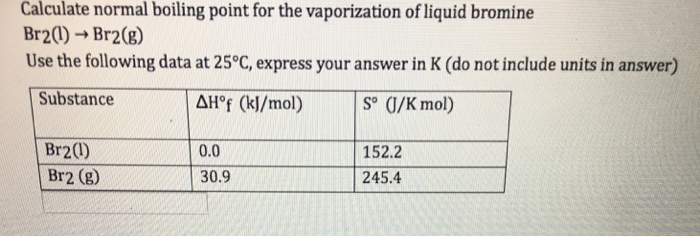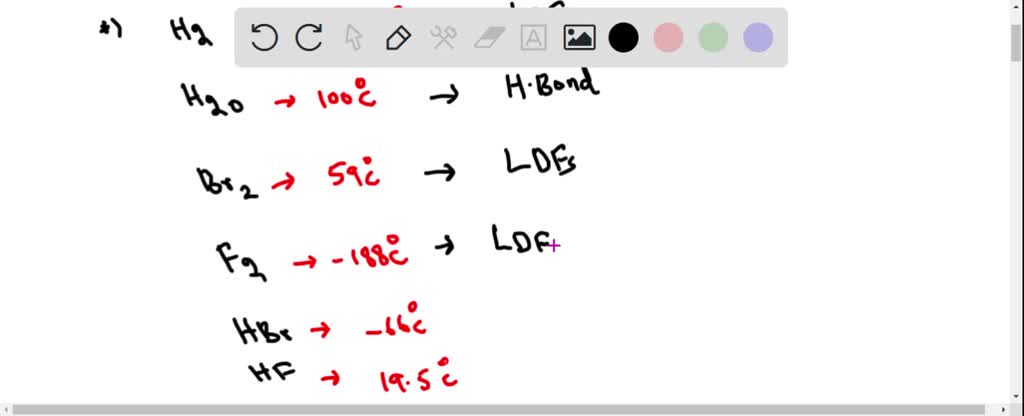
SOLVED: 5.Search the boiling point of H2, H2O, Br2, F2, HBr y HF. Explain this behavior based on the intermolecular theory.

SOLVED: Identifythe moelcule you expect to have the highest boiling point? OA F2| 0 B Cl2 C Br2 0D. I2 OE All of the above have the same boiling point
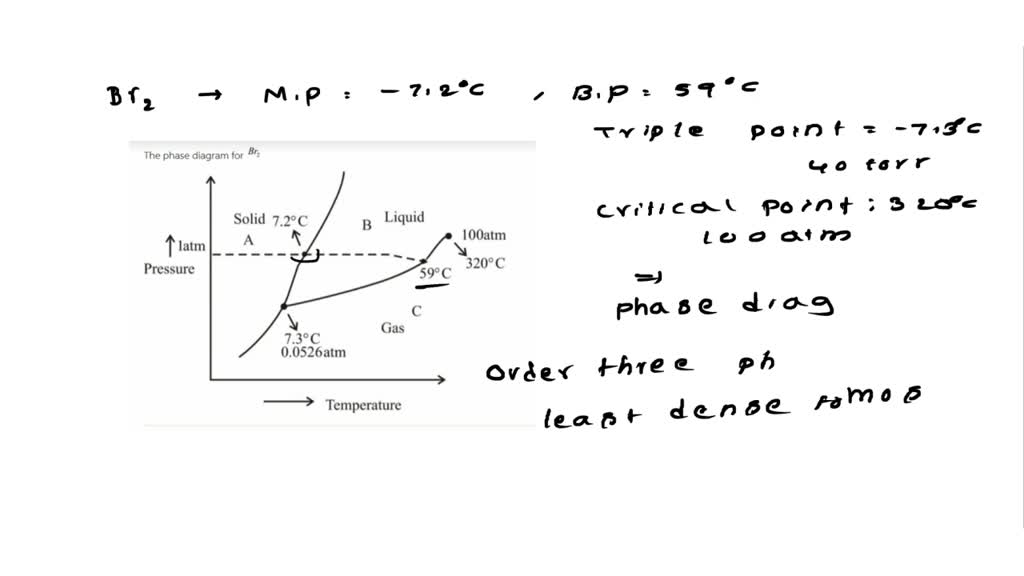
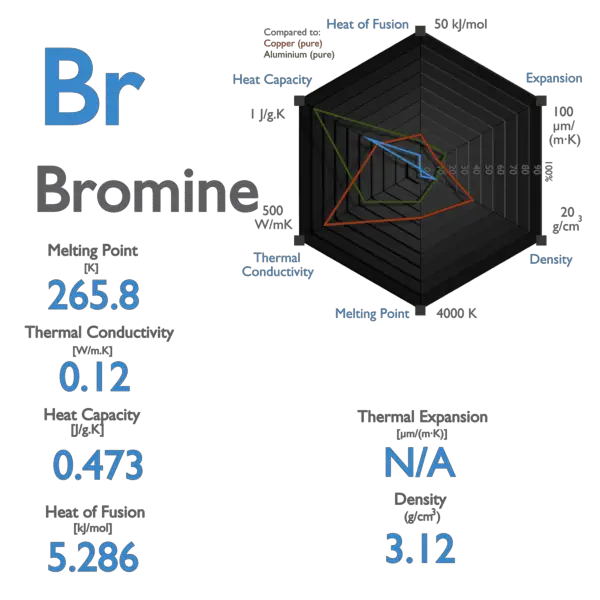
SOLVED: Bromine (Br2) has a normal melting point of – 7.2°C and a normal boiling point of 59°C. The triple point of Br2 is – 7.3°C and 40 mm Hg, and the

What will be the boiling point of bromine when 174.5mg of octa - atomic sulphur is added to 78g of bromine? Kb for Br2 is 5.2Kmol^-1 kg and b.pt of Br2 is