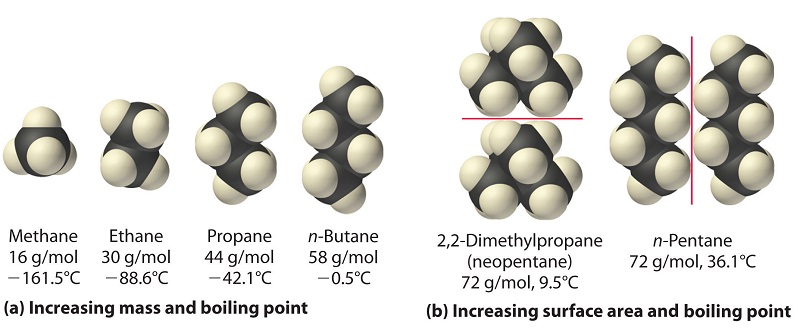
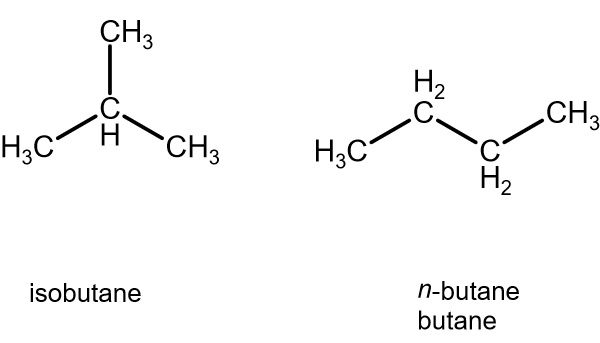
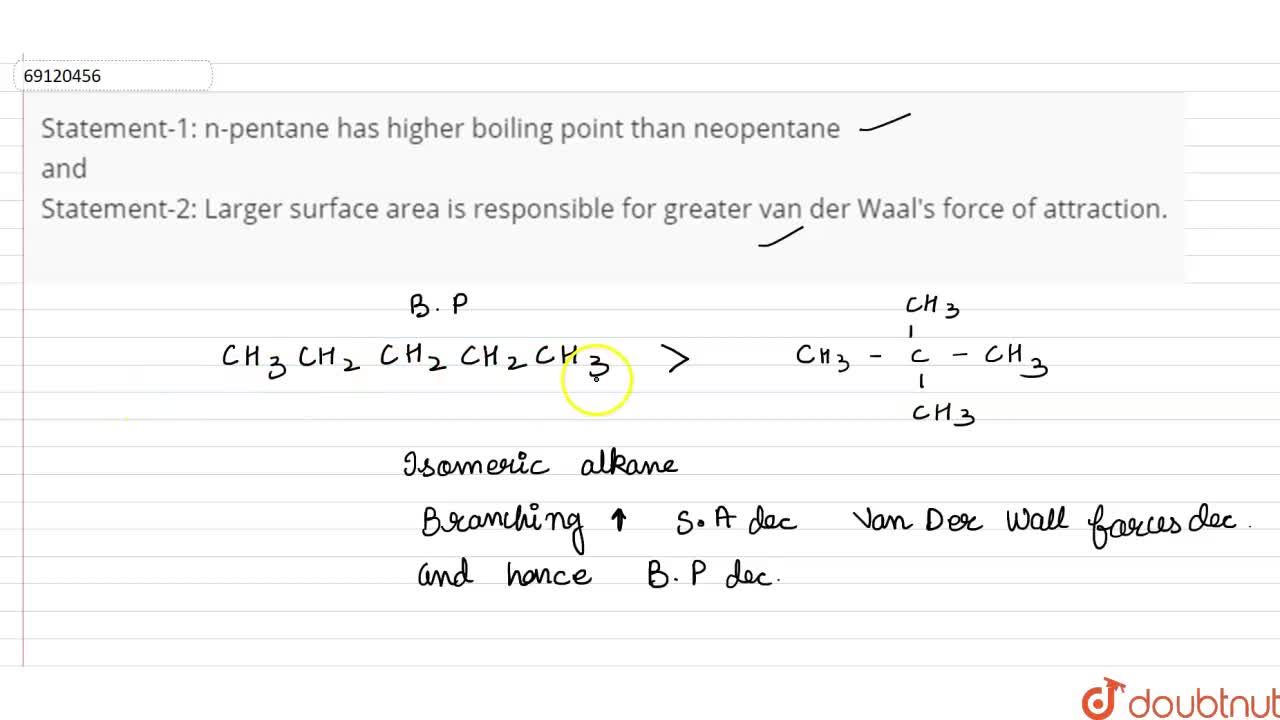
SOLVED: 1)The following information is given for n-pentane, C5H12, at 1atm: boiling point = 36.2 °C Hvap(36.2 °C) = 25.8 kJ/mol specific heat liquid = 2.28 J/g°C At a pressure of 1

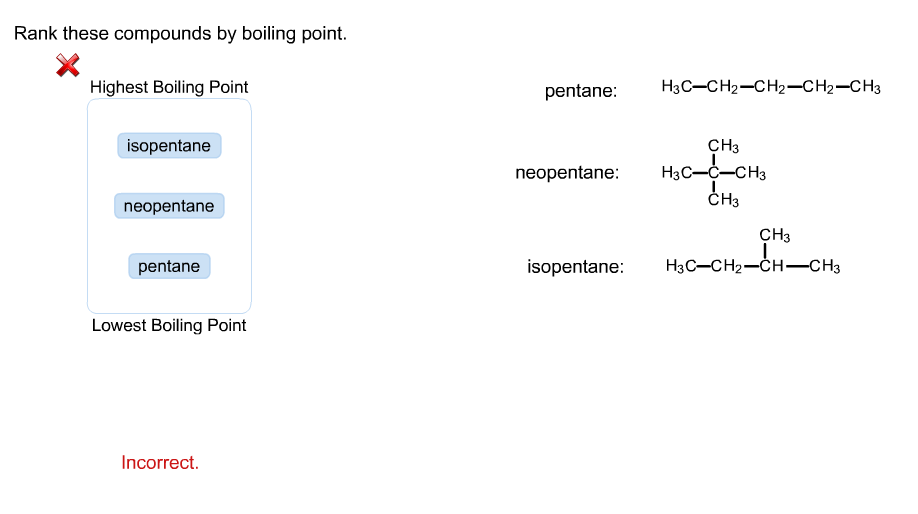
Rank these compounds from highest to lowest boiling point. a. pentane b. neopentane c. isopentane | Homework.Study.com
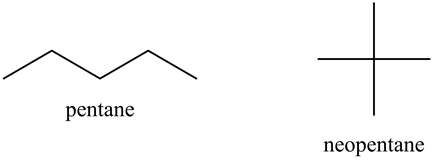
Rank these compounds by boiling point from highest to lowest boiling point: pentane, neopentane, hexane - Home Work Help - Learn CBSE Forum

Arrange the following compounds in the descending order of their boiling pointsa) n - pentaneb) isopentanec) neopentane

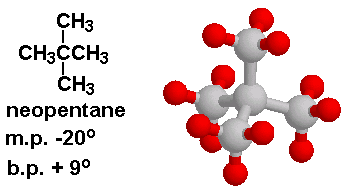
why neopentane has higher melting point than n pentane - Chemistry - Chemical Bonding and Molecular Structure - 13416933 | Meritnation.com