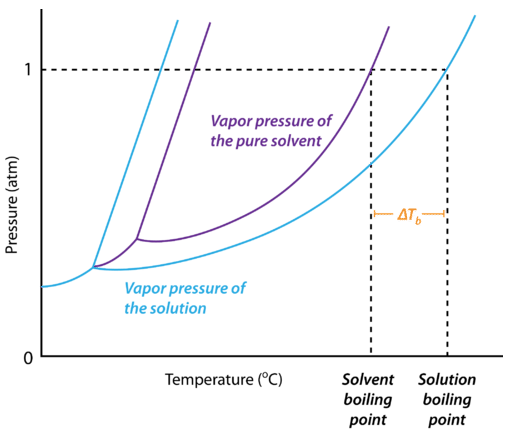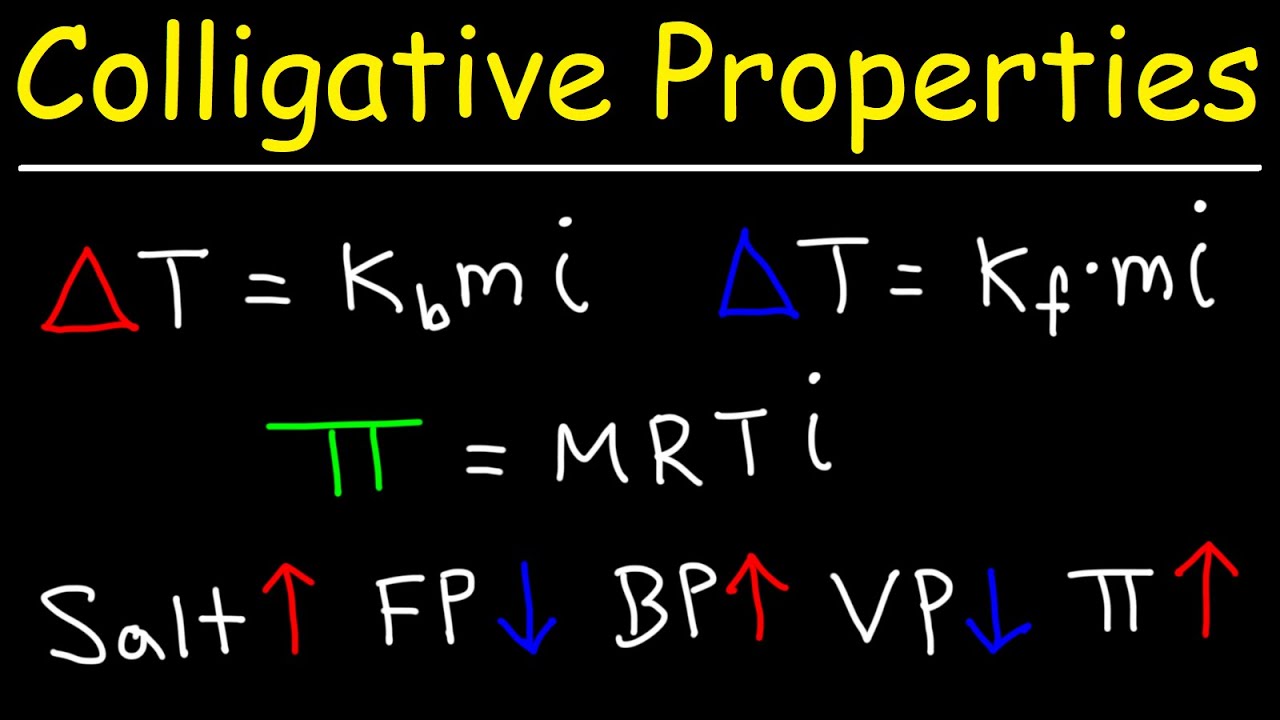
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure - YouTube
Calculate the boiling point of solution when 4 g of MgSO4 (M = 120 g mol^-1) was dissolved in 100 g of water assuming - Sarthaks eConnect | Largest Online Education Community

OneClass: Calculate the normal boiling point of a compound that has a vaporpressure of 500 torr at 20...
57. calculate bp of solution cntaining 25g urea 25g thio urea in 500g of chloroform boiling point of pure chloroform is 61.2 degree celsius Kb = 3.63

Calculate the freezing point and the boiling point at 1 atmosphere of a solution containing 30 g cane sugar (molecular mass 342 ) and 150 g water.Given : Kb = 0.513 and Kf = 1.86

Calculation the boiling point of a 1M aqueous solution (density 1.04 g mL^-1 )of potassium chloride (Kb for water = 0.52 K kg mol^-1 , Atomic masses: K = 39u, Cl =
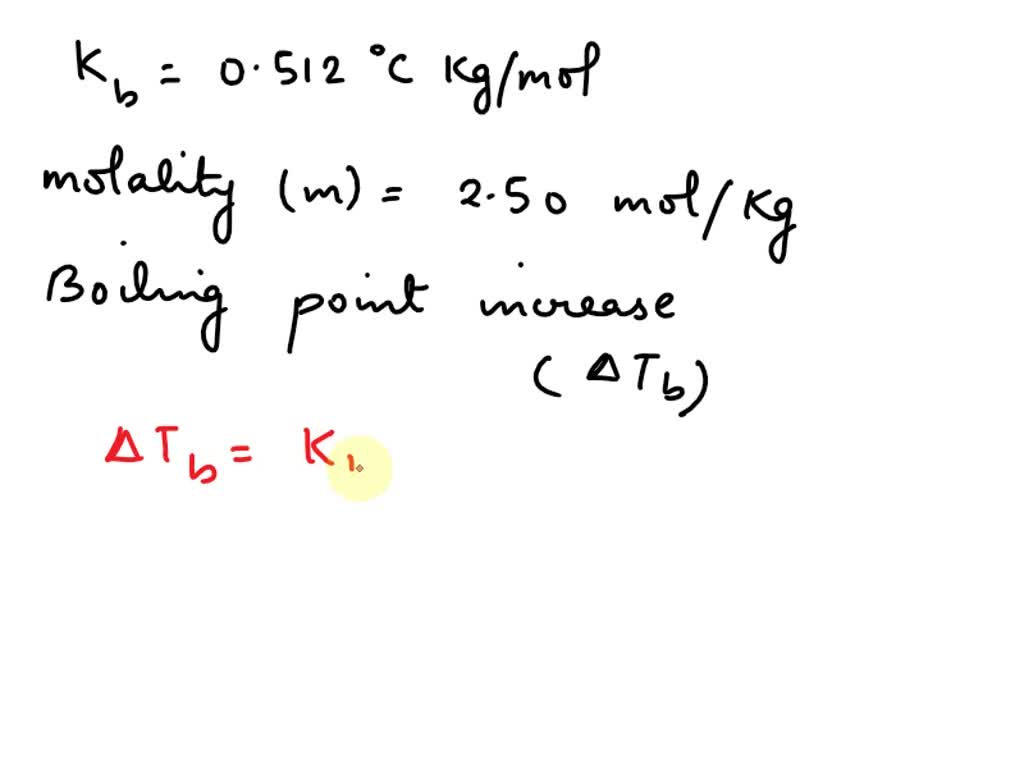
SOLVED: Given that water's boiling point is 100ºC and the elevation constant (Kb) is 0.512 ºCkg/mol, calculate the boiling point increase for a 2.50 molal solution (moles/kg) of aqueous NaCl. Assume ideal
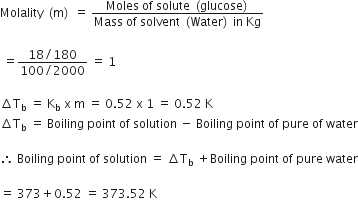
The boiling point of pure water is 373K. Calculate the boiling point of an aqueous solution containing 18 gms of glucose (MW = 180) in 100 gms of water. Molal elevation constant

Assuming 100% dissociation, calculate the freezing point and boiling point of 2.13 m Na_2SO_4(aq). | Homework.Study.com