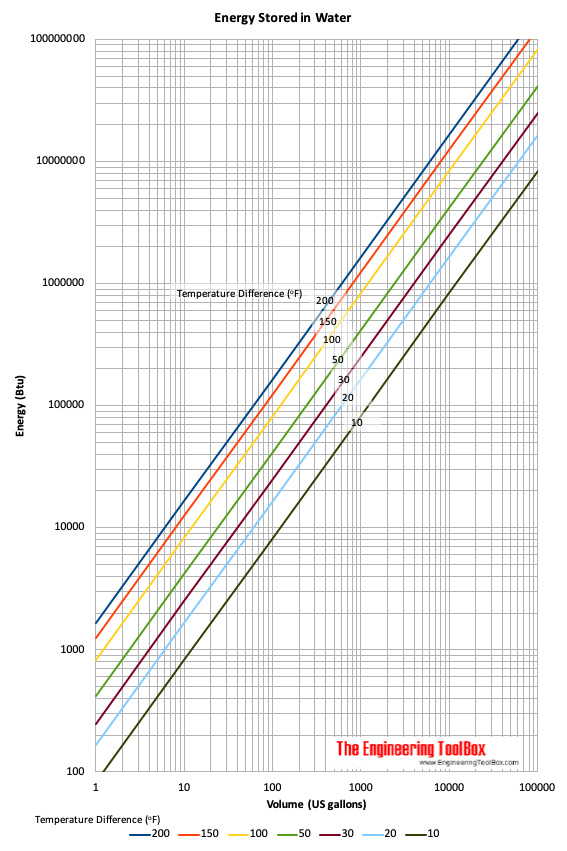
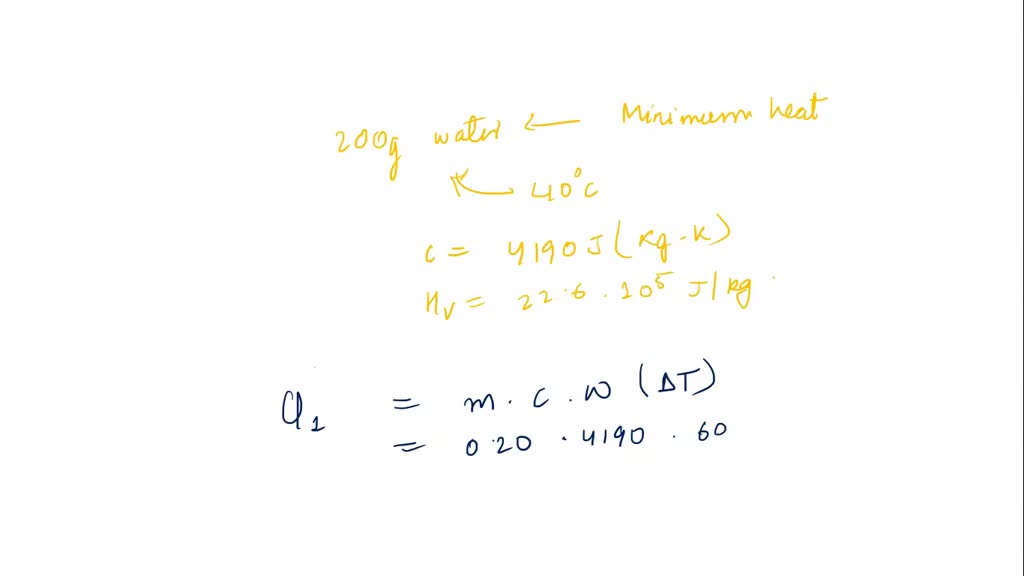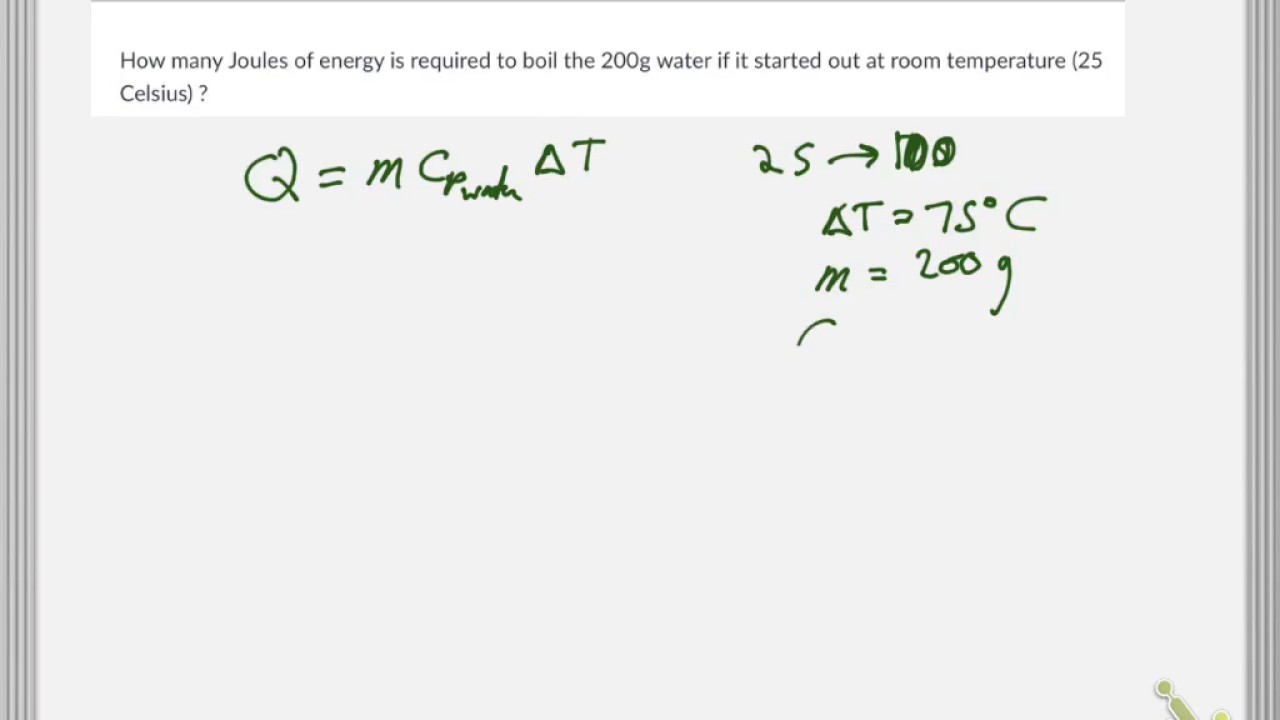How much power do I need in watts or volts to heat 1 litre of water from 0 to 100 and/or 110 degrees Celcius in less than 5 minutes? - Quora

The heat energy required to vaporize 5 kg of water at 373 K is nearly (Latent heat of vaporization Lv = 2270 J )

Heat and Temperature Heat is a form of energy, and is measured in Joules (J). Temperature is different from heat. Temperature is a measure of how hot or. - ppt video online

Find how long it takes to bring a cup of water to boiling temperature in a 600 watt microwave 1-28 - YouTube

Question Video: Finding the Time a Device Is Used for given the Useful Energy Output, Efficiency, and Total Power Output | Nagwa
Find the heat energy required to boil 5 kg of water iff its initial temperature is 30^(@)C (specific heat of water is 4200 J kg^(-1)" "k^(-1) and boiling point is 100^(@)C)

Calorie (energy) Calculations A calorie is defined as the amount of energy it takes to raise the temperature of one gram of water by one degree Celsius. - ppt download

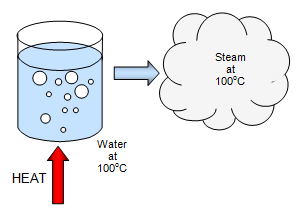
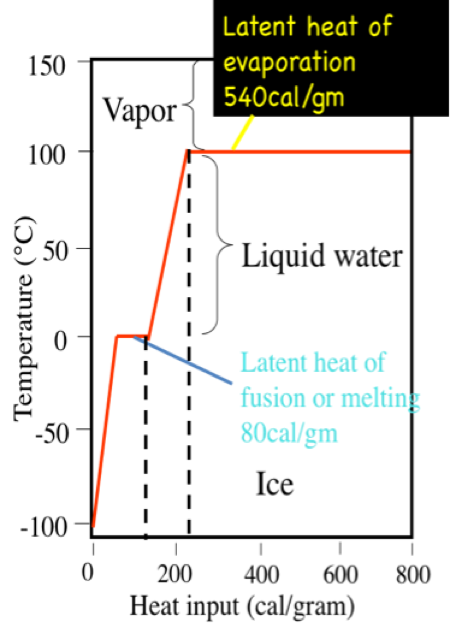
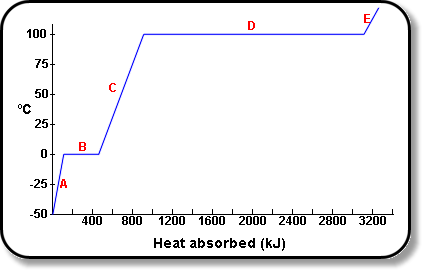
Question Video: Finding the Amount of Energy Needed to Change the State of Water from Liquid to Gas | Nagwa