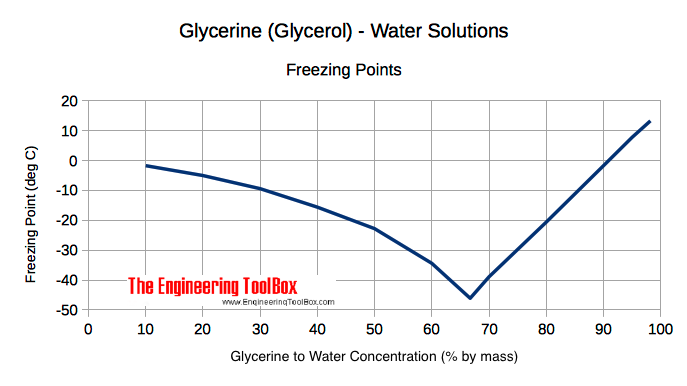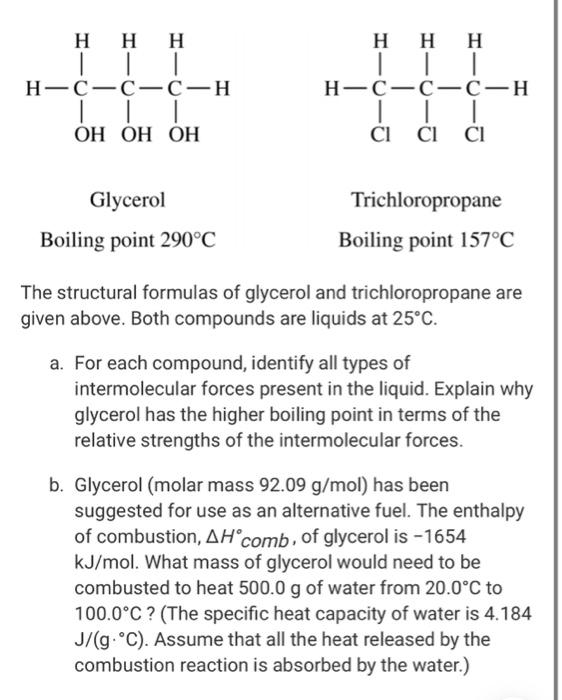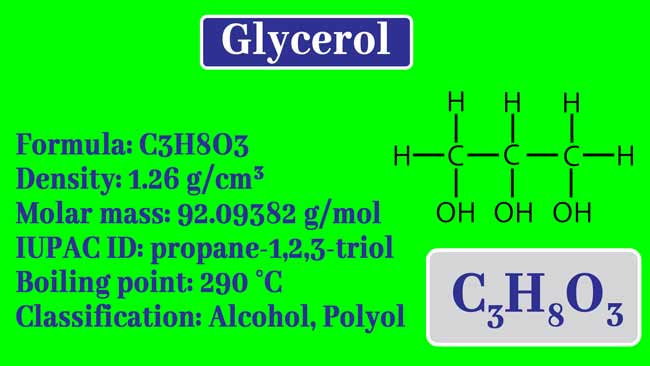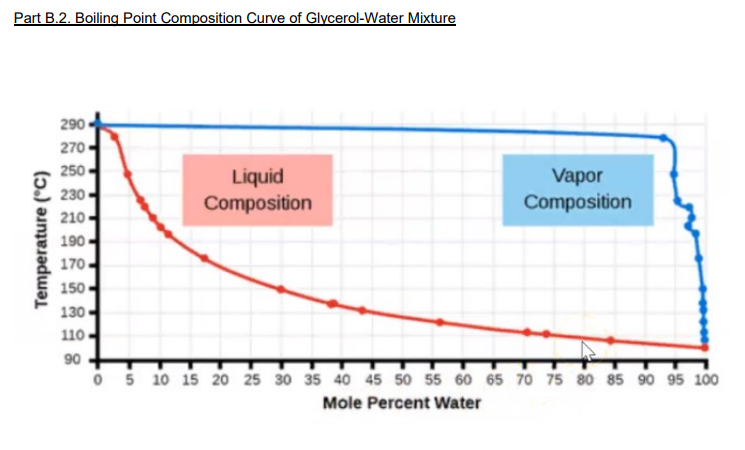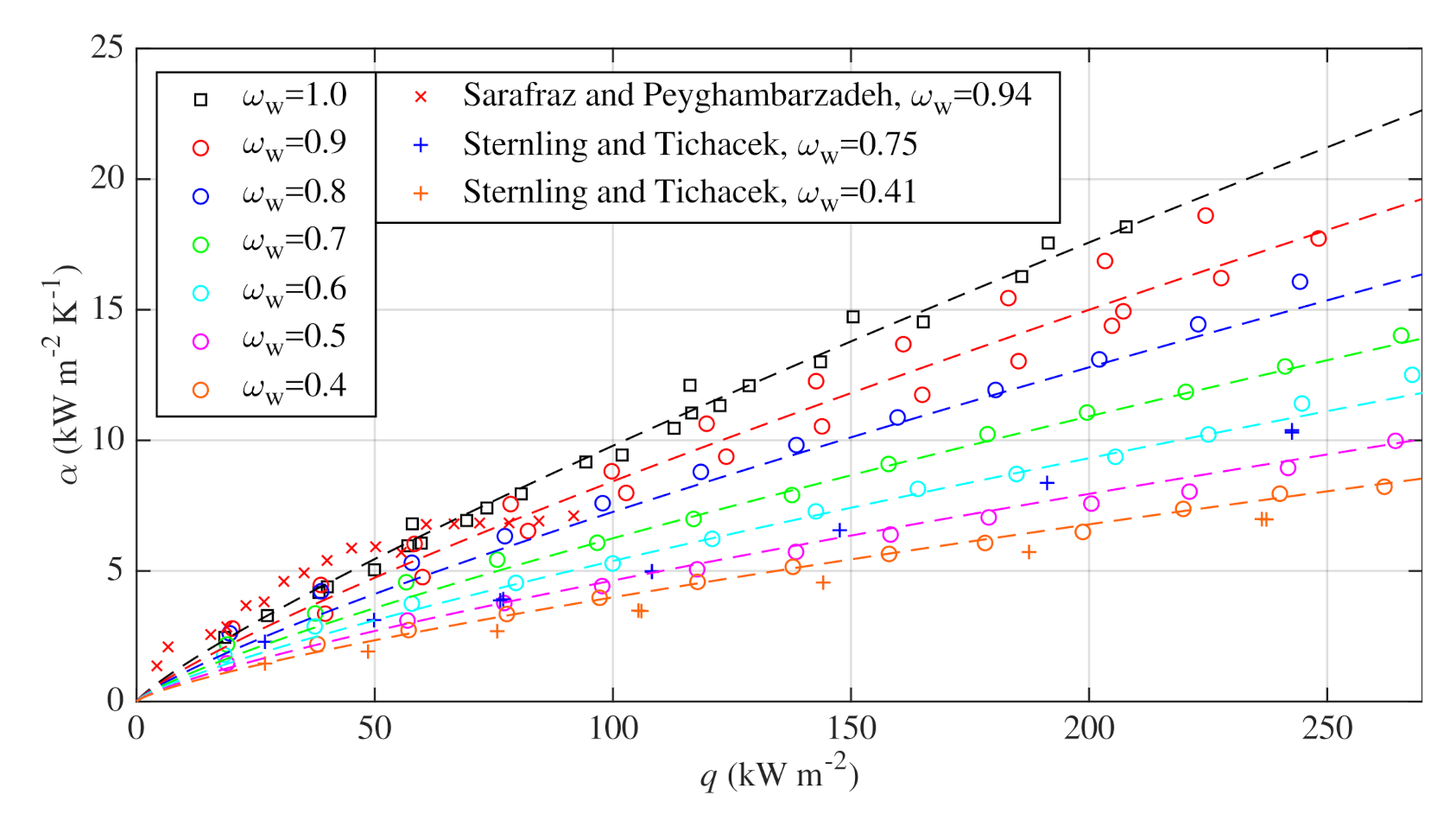
Processes | Free Full-Text | Pool Boiling Heat Transfer Coefficients in Mixtures of Water and Glycerin
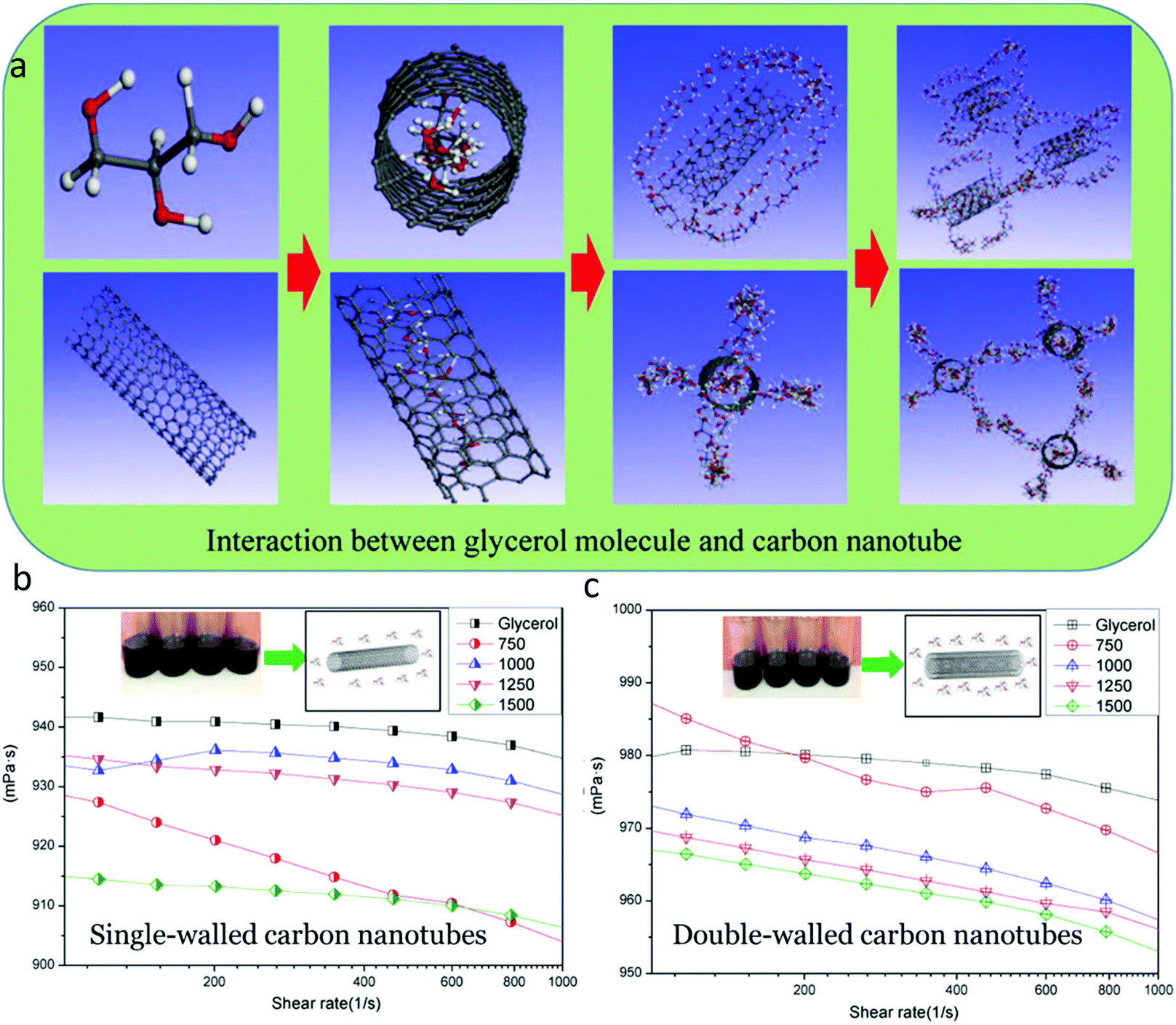
Glycerol in energy transportation: a state-of-the-art review - Green Chemistry (RSC Publishing) DOI:10.1039/D1GC02597J
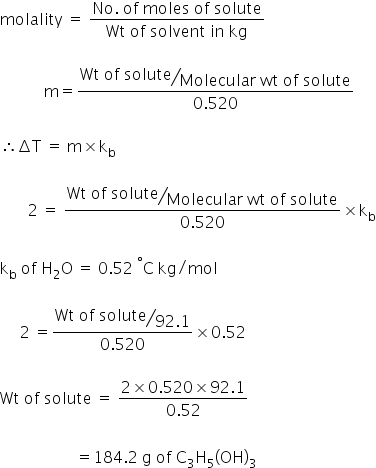
calculate the number of grams of glycerol c3h5 oh 3 mw 921g mol that must be dissolved in5 520 gm grams of water to raise the boiling point to 102000c 00nc4oii -Chemistry -

Vapor–Liquid Equilibrium of Water + Ethanol + Glycerol: Experimental Measurement and Modeling for Ethanol Dehydration by Extractive Distillation | Journal of Chemical & Engineering Data

Glycerol decomposes at its boiling point (563 K) . Discuss a method which can used for its purification.

A solution of glycerol `(C-(3)H_(8)O_(3)` , molar mass = 92 g `mol^(-1)` iin water was prepared by - YouTube
EFFECTS OF ADDITION GLYCEROL CO-PRODUCT OF BIODIESEL IN THE THERMOPHYSICAL PROPERTIES OF WATER-GLYCEROL SOLUTION APPLIED AS SECO

SOLVED: Using the data provided below, calculate the boiling point (in oC) of a 0.22 molal solution of glycerol (C3H8O3) in ethanol (C2H6O). ethanol normal boiling point = 78.4 oC; Kb (oC/molal) =