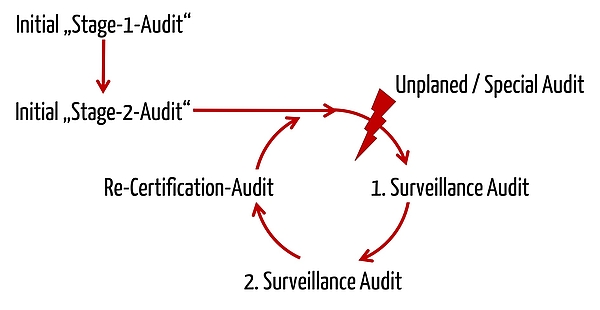
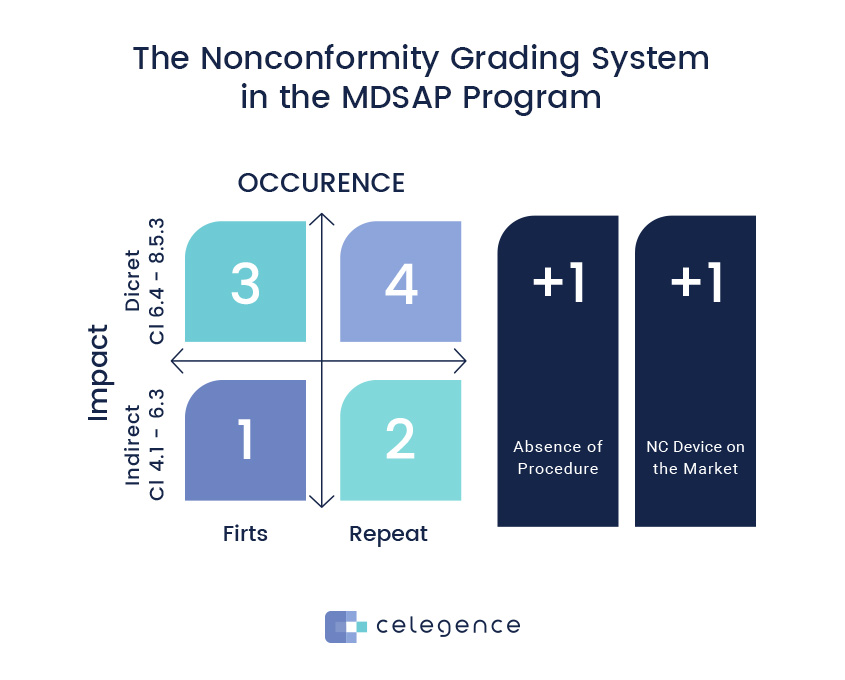
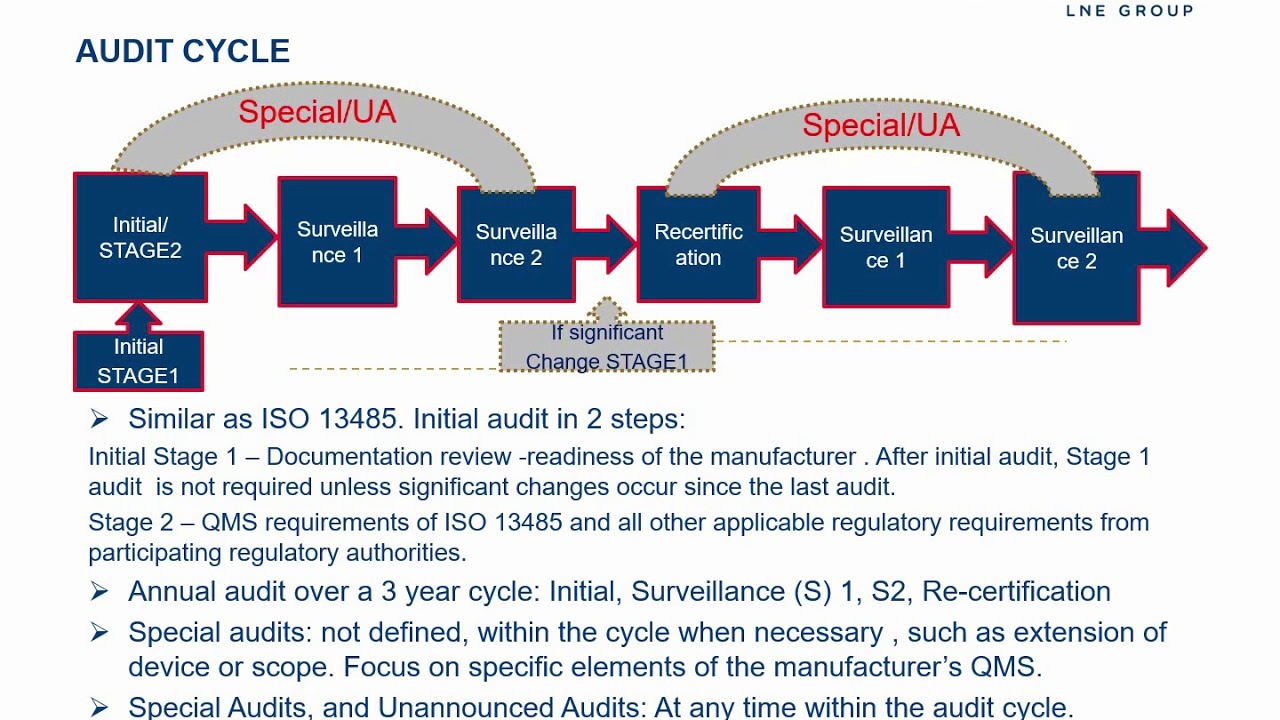
Medical Device Single Audit Program (MDSAP) – Chapters 1 to 4 - LearnGxP: Accredited Online Life Science Training Courses
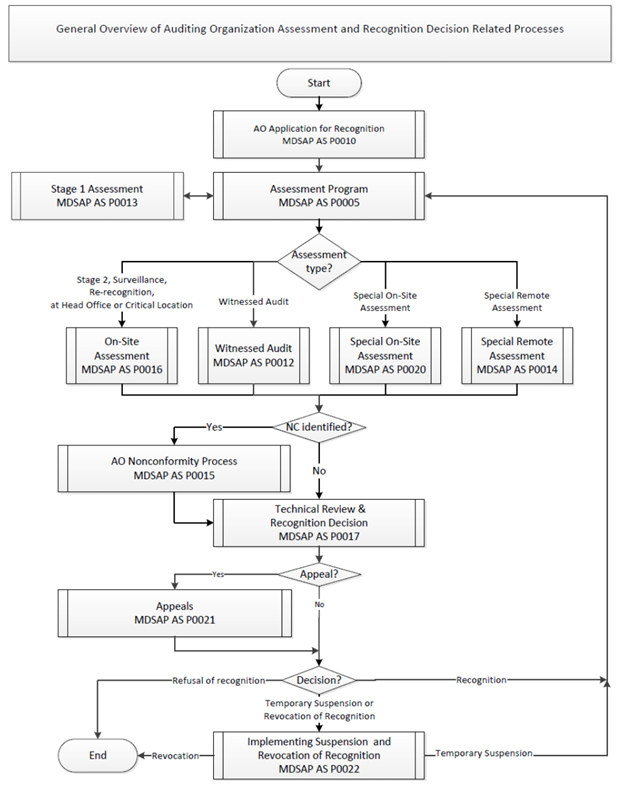
Guidance Document: Requirements in the Recognition Process for Medical Device Single Audit Program (MDSAP) Auditing Organizations - Canada.ca

Freyr Medical Devices Regulatory Services on LinkedIn: Medical Device Single Audit Program (MDSAP) - Audit Processes

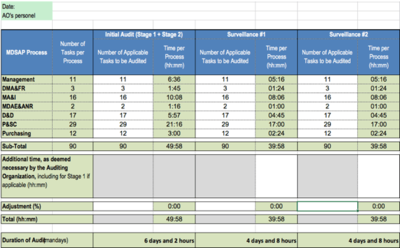

![Complete MDSAP Guide: Medical Device Single Audit Program [Video] Complete MDSAP Guide: Medical Device Single Audit Program [Video]](https://easymedicaldevice.com/wp-content/uploads/2018/12/NGE-filled-Summary-2-1024x415.jpg)