How hard is it to melt diamond? Can I make profit melting many little diamonds into one big diamond? I've heard scientists have melted diamond at high temperature and pressures with strong
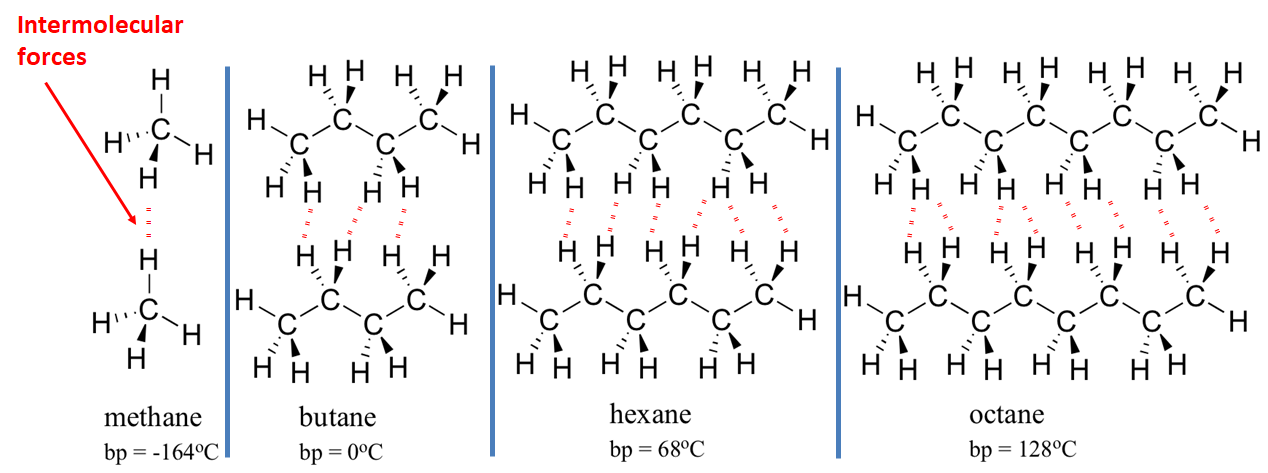
1:48 explain why the melting and boiling points of substances with simple molecular structures increase, in general, with increasing relative molecular mass - TutorMyself Chemistry
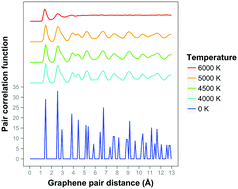
The initial stages of melting of graphene between 4000 K and 6000 K - Physical Chemistry Chemical Physics (RSC Publishing)

Measurements of the melting point of graphite and the properties of liquid carbon (a review for 1963–2003) - ScienceDirect

Melting point (mp) - solid to liquid Boiling point (bp) - liquid to gas Volatility - how easily it is converted to gas Conductivity (conducts. - ppt download

Measurements of the melting point of graphite and the properties of liquid carbon (a review for 1963–2003) - ScienceDirect

Formula: Ir Melting Point: 2410ºC Boiling Point: 4130ºC State: solid Electrical conductivity: conductor Magnetism: non magnetic. - ppt download