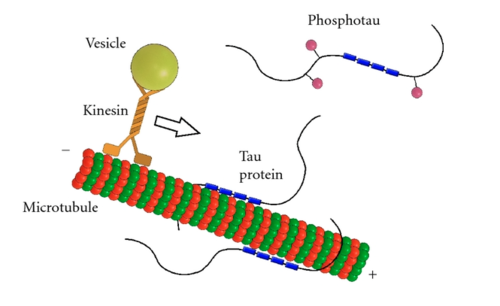
Heterotypic electrostatic interactions control complex phase separation of tau and prion into multiphasic condensates and co-aggregates | PNAS

Tau K321/K353 pseudoacetylation within KXGS motifs regulates tau–microtubule interactions and inhibits aggregation | Scientific Reports
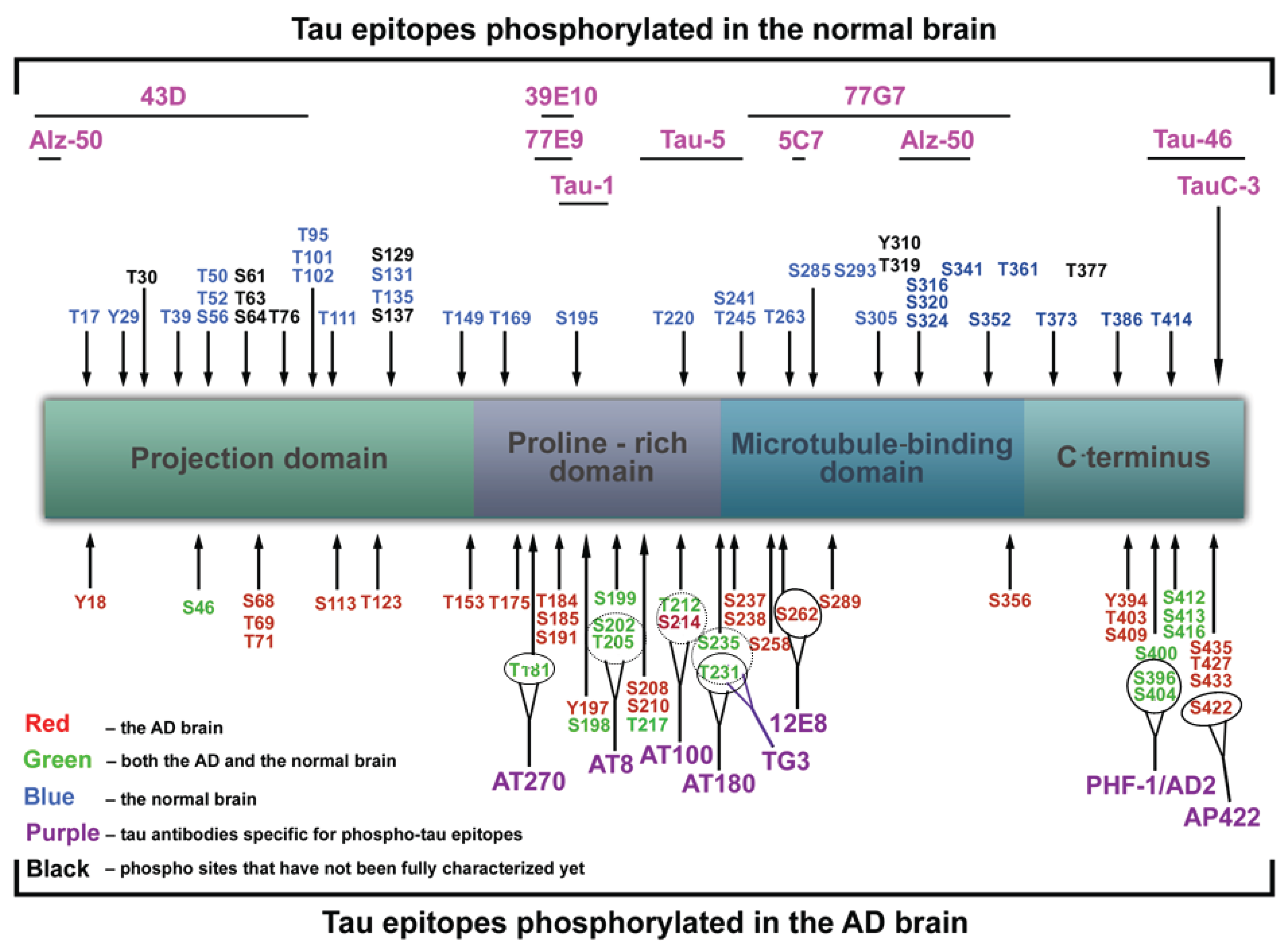
Biomolecules | Free Full-Text | Tau Protein Hyperphosphorylation and Aggregation in Alzheimer's Disease and Other Tauopathies, and Possible Neuroprotective Strategies
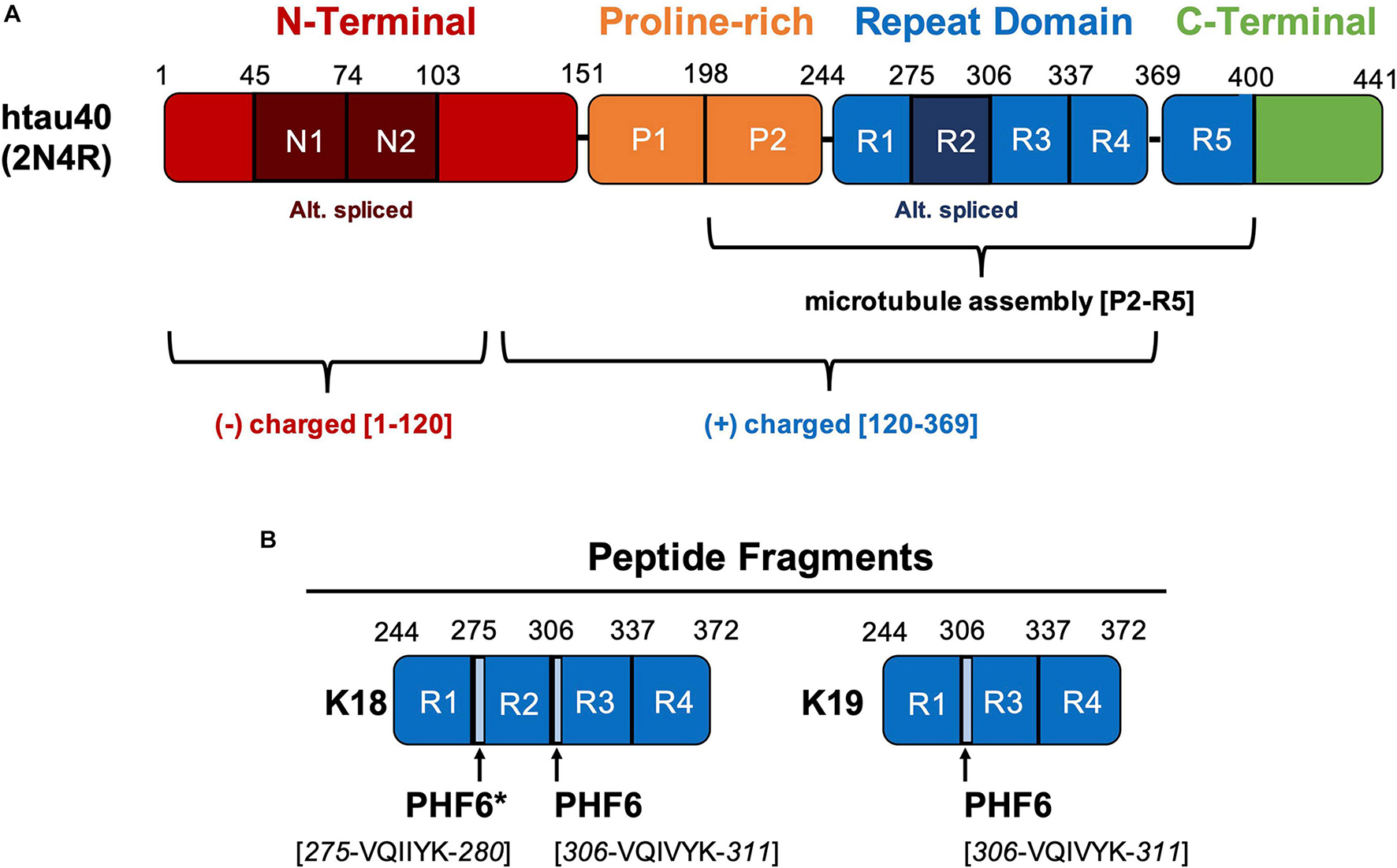
Liquid–liquid phase separation of the microtubule-binding repeats of the Alzheimer-related protein Tau | Nature Communications
Biomolecules | Free Full-Text | Tau Protein Hyperphosphorylation and Aggregation in Alzheimer's Disease and Other Tauopathies, and Possible Neuroprotective Strategies

Figure 4. | Reversible Paired Helical Filament-Like Phosphorylation of Tau Is an Adaptive Process Associated with Neuronal Plasticity in Hibernating Animals | Journal of Neuroscience
Molecules | Free Full-Text | Aβ and Tau Interact with Metal Ions, Lipid Membranes and Peptide-Based Amyloid Inhibitors: Are These Common Features Relevant in Alzheimer’s Disease?
Intersection of pathological tau and microglia at the synapse | Acta Neuropathologica Communications | Full Text

Specific Degradation of Endogenous Tau Protein and Inhibition of Tau Fibrillation by Tanshinone IIA through the Ubiquitin–Proteasome Pathway | Journal of Agricultural and Food Chemistry

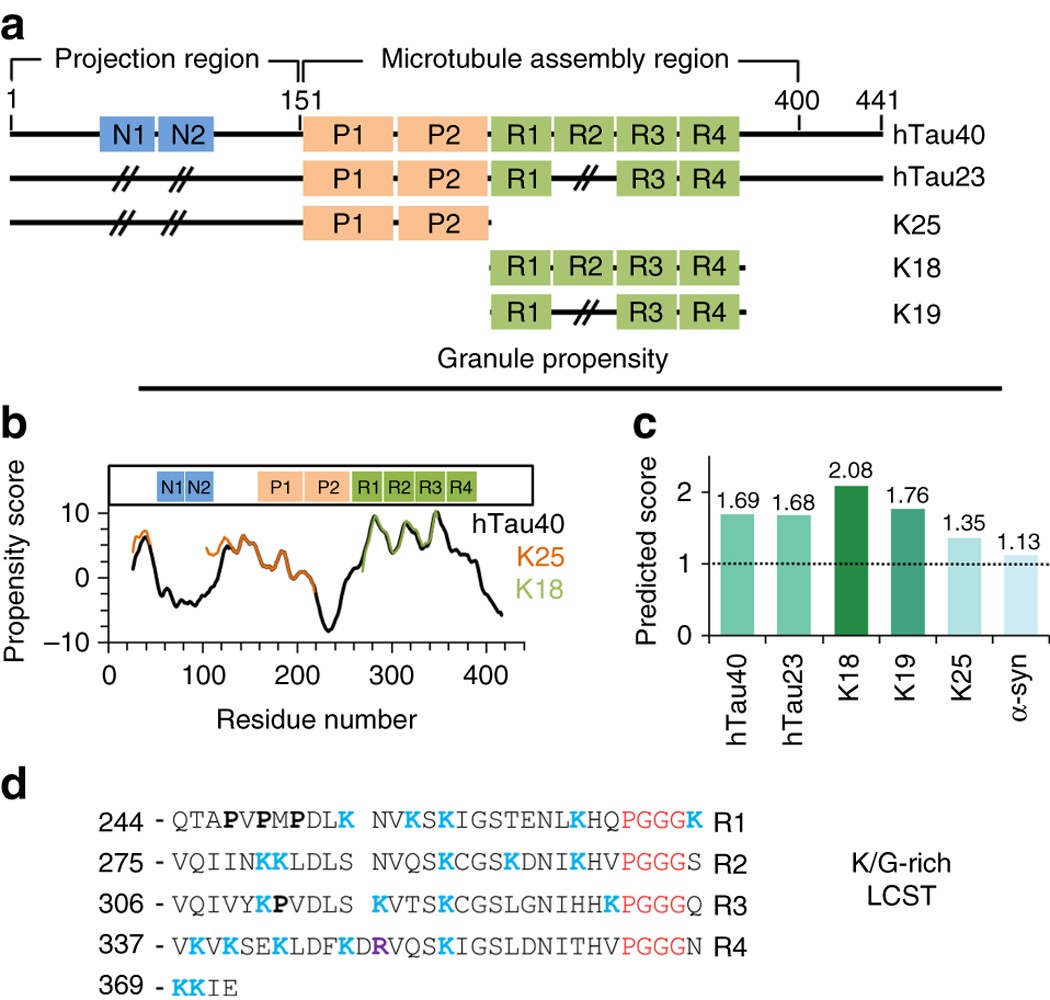
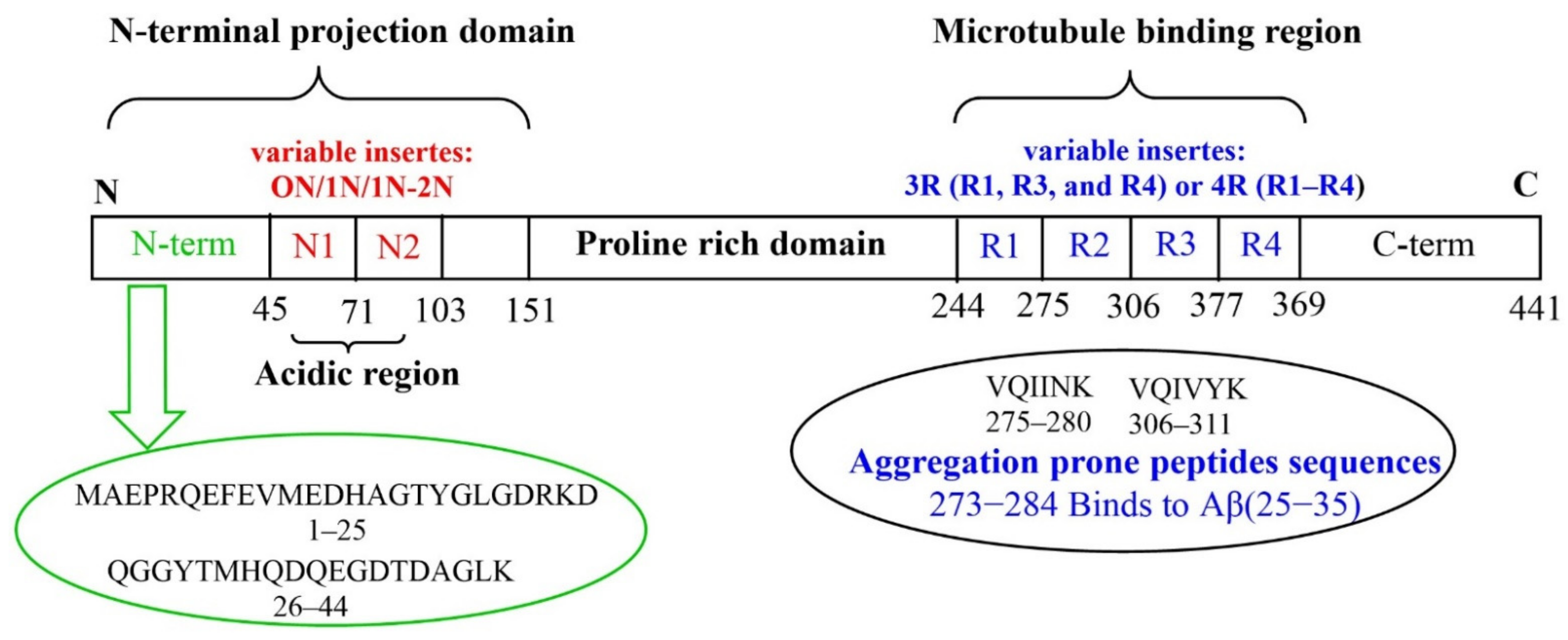
Removal of Pattern-breaking Sequences in Microtubule Binding Repeats Produces Instantaneous Tau Aggregation and Toxicity - ScienceDirect

Phosphorylation of Tau Protein Associated as a Protective Mechanism in the Presence of Toxic, C-Terminally Truncated Tau in Alzheimer's Disease | IntechOpen

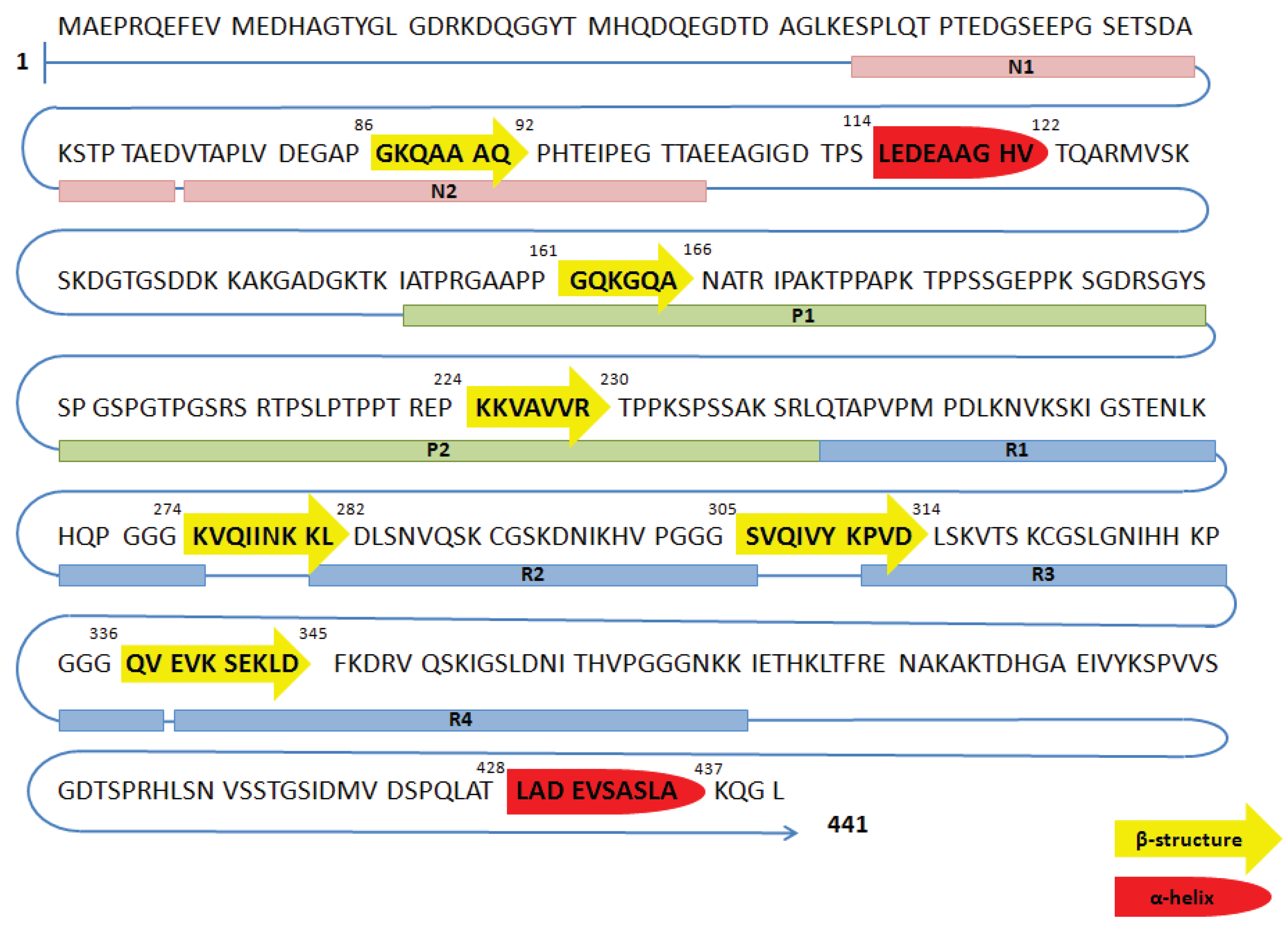
Amino acid sequence of the longest tau isoform (441 amino acids). N1... | Download Scientific Diagram

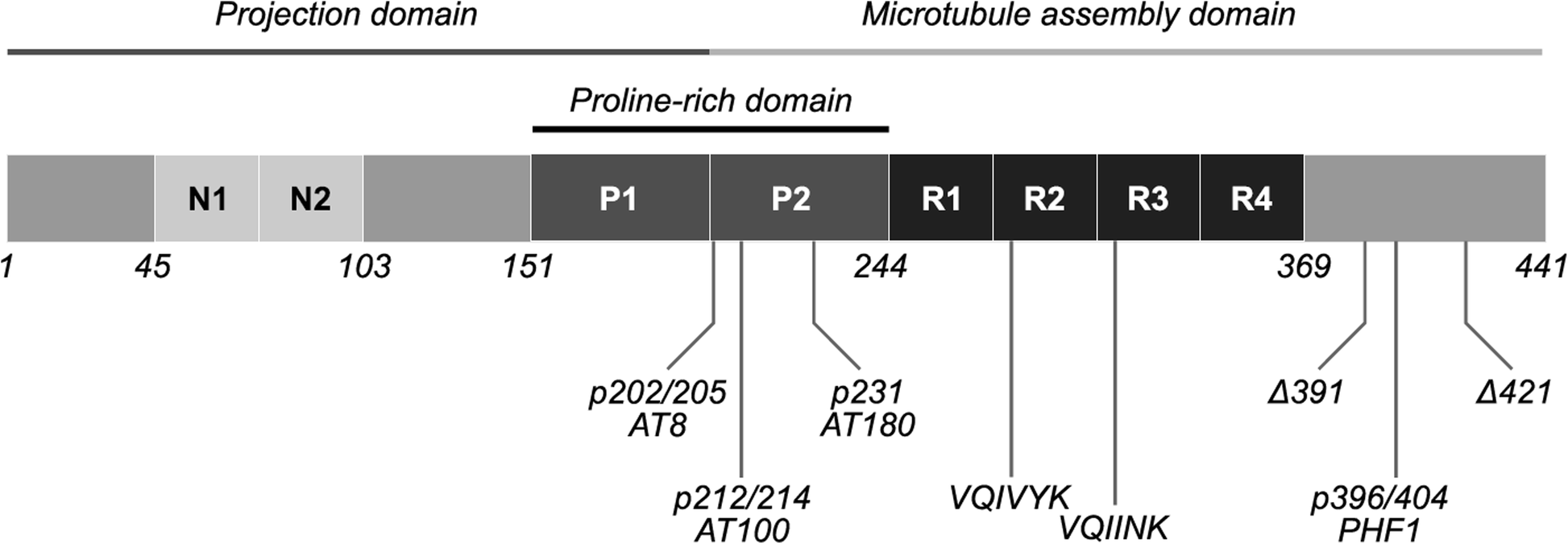
Frontiers | Phospho-Tau Bar Code: Analysis of Phosphoisotypes of Tau and Its Application to Tauopathy

Identification of key amino acids responsible for the distinct aggregation properties of microtubule‐associated protein 2 and tau - Xie - 2015 - Journal of Neurochemistry - Wiley Online Library

A mechanistic model of tau amyloid aggregation based on direct observation of oligomers | Nature Communications

We reported the full-length amino acid sequence of the human and mouse... | Download Scientific Diagram

Amino acid sequence of the PHF-Tau protein with the observed b-strand... | Download Scientific Diagram

Structure-Based Design and Biological Evaluation of Novel Caspase-2 Inhibitors Based on the Peptide AcVDVAD-CHO and the Caspase-2-Mediated Tau Cleavage Sequence YKPVD314 | ACS Pharmacology & Translational Science