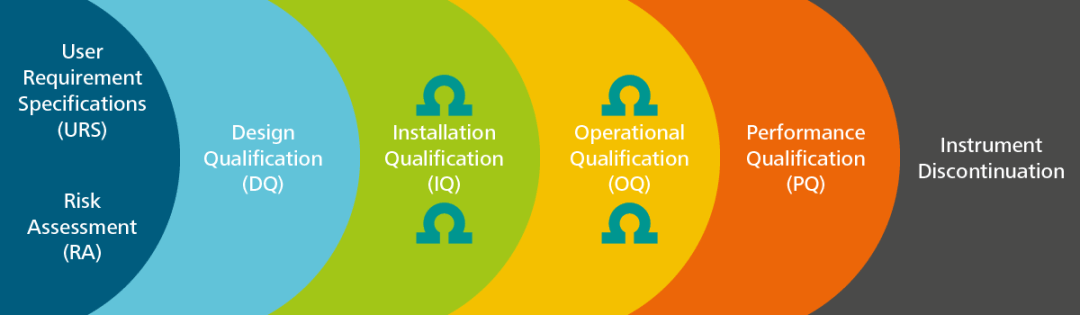
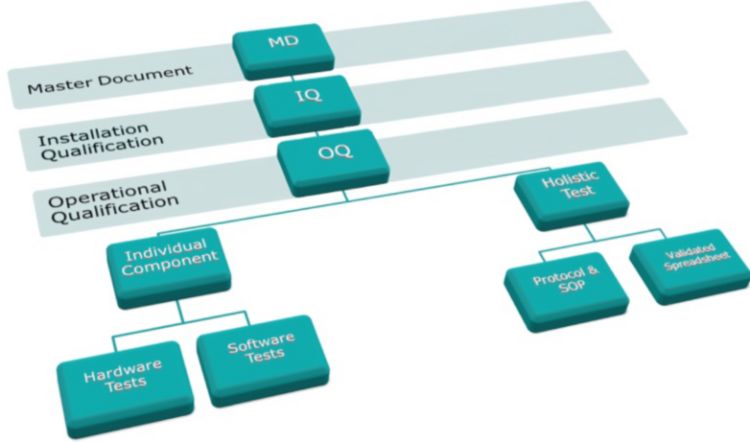
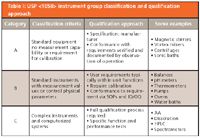
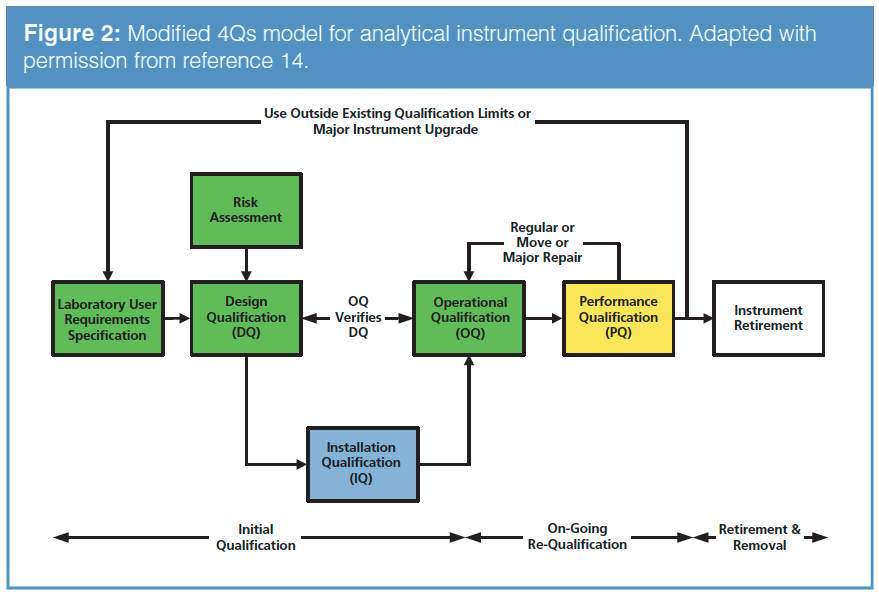
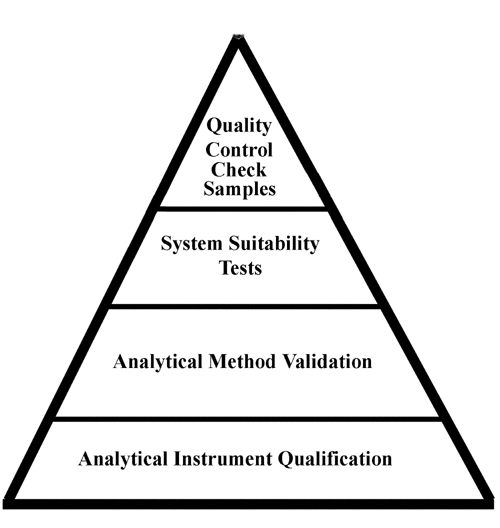
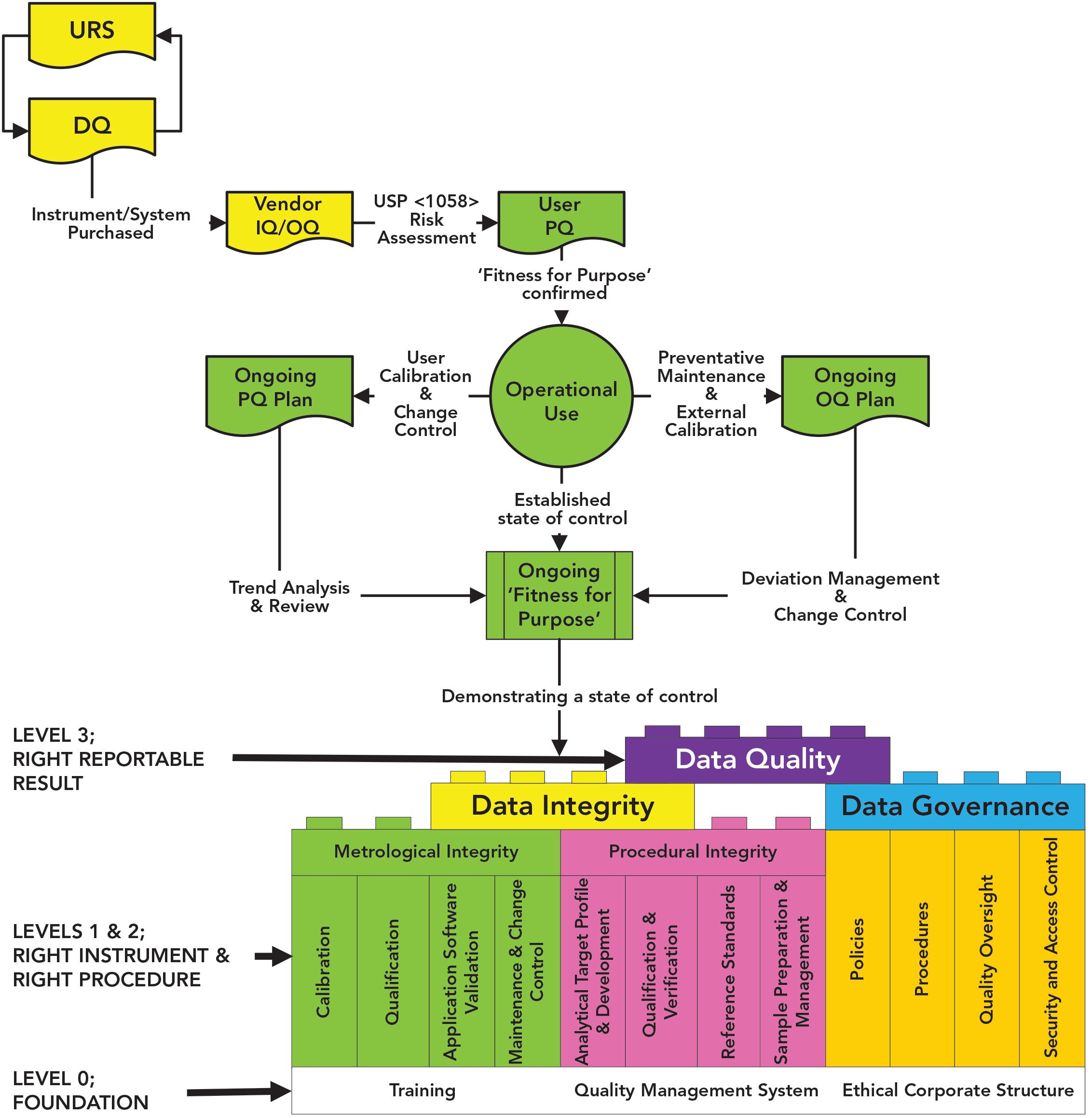
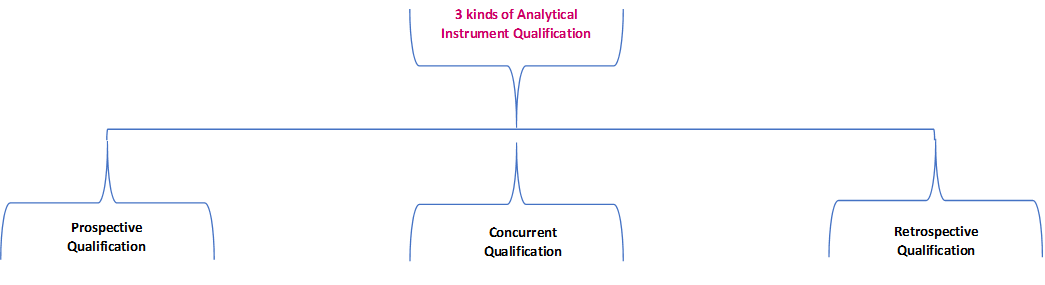
pi on Twitter: "#AIQ or Analytical Instrument Qualification forms the basis of #dataquality - learn more about the #USP Chapter 1058 revision - https://t.co/qYglXPhrac #Regulatory #LifeSciences https://t.co/Fpx9YZDTDx" / Twitter
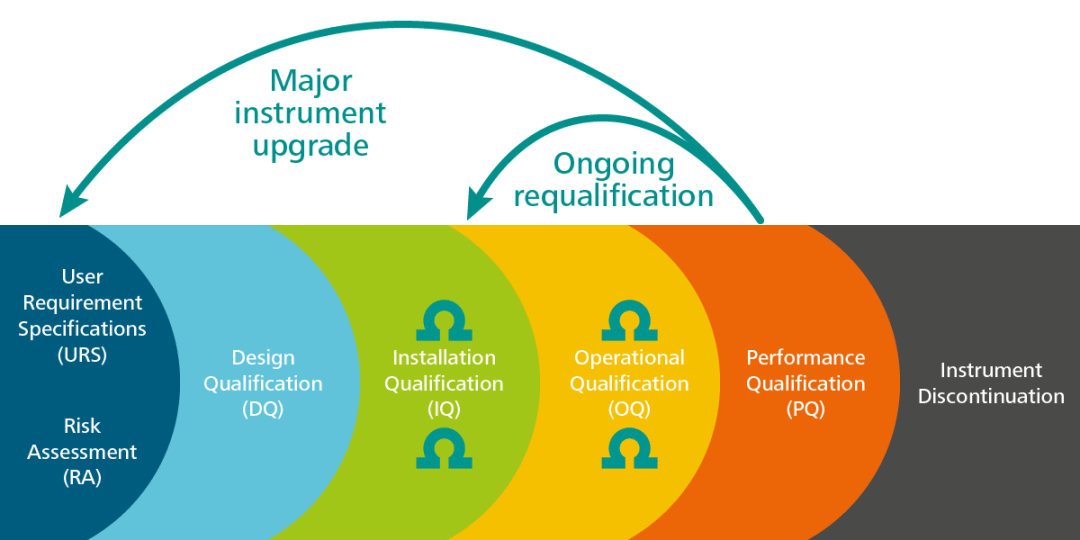
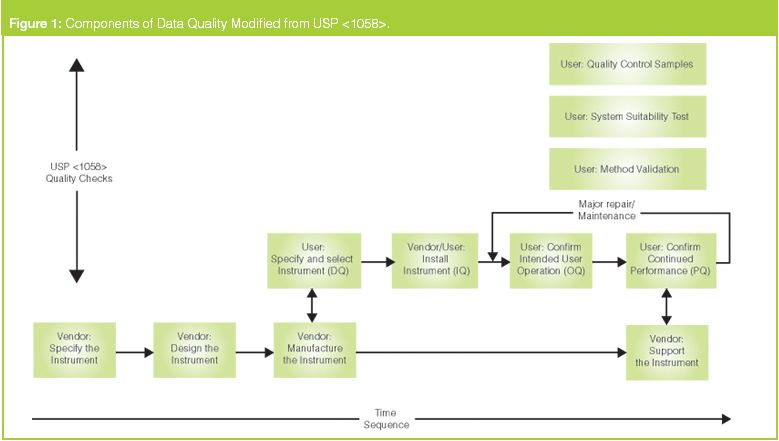
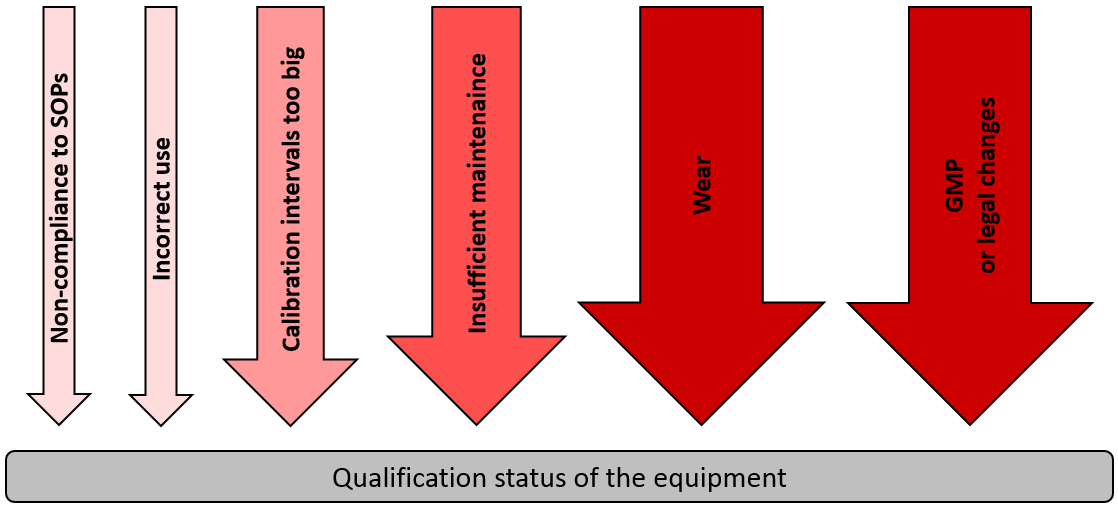
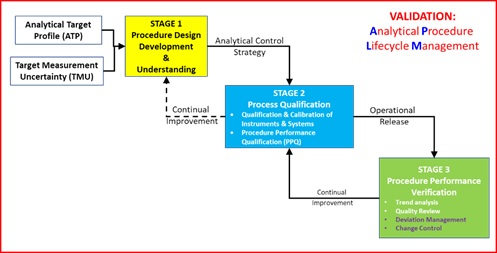
November DDG - USP<1058> Analytical Instrument Qualification Revision and Its Impact on Dissolution Apparatus Qu